About AtoCap
 AtoCap’s team combines world-class academic, clinical and commercialisation expertise and has a strong track record of successfully translating highly innovative technologies into commercial opportunities that generate substantive income and impact endpoints for stakeholders and investors.
AtoCap’s team combines world-class academic, clinical and commercialisation expertise and has a strong track record of successfully translating highly innovative technologies into commercial opportunities that generate substantive income and impact endpoints for stakeholders and investors.
The team initially developed from within University College London’s Encapsulation Research Group and is based on a novel implementation of electro-hydrodynamic processing for the preparation of a wide range of encapsulated structures via a clean, efficient, one-step route.
Our Technology
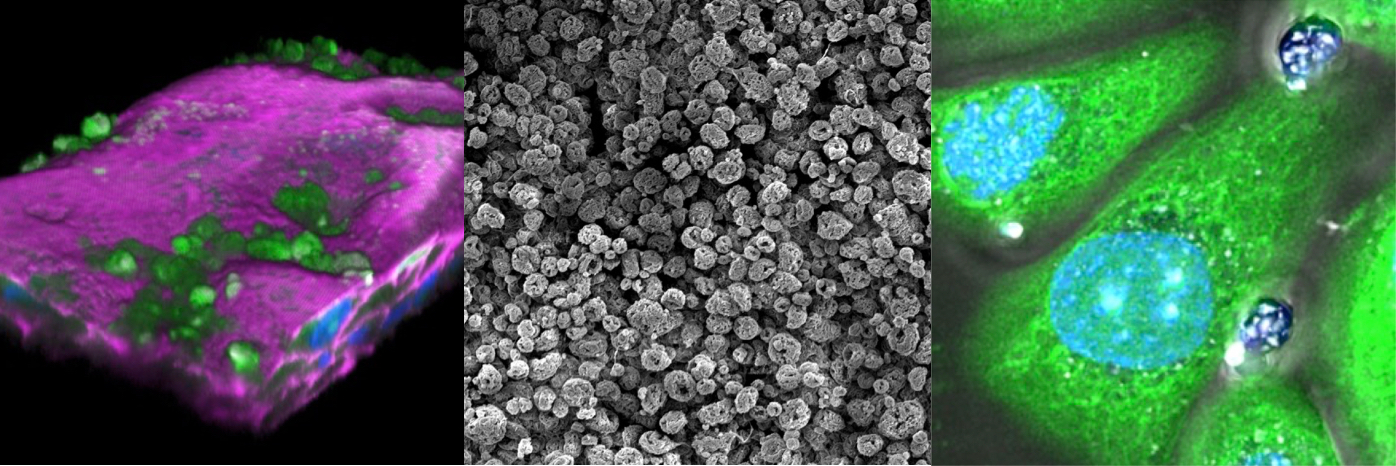
Microencapsulation: AtoCap Technology enables the encapsulation of patient-specific combinations of generic drugs such as antibiotics and chemotherapeutics into a multi-layered capsule.
Our first product, CapFuran®, enables time-release killing of a variety of patient-derived uropathogenic bacterial species, including E. coli, by a well-known and frequently-used generic antibiotic encapsulated using innovative methodology. In pre-clinical experiments, CapFuran® eradicated bacteria buried deep within a multi-layered human bladder organoid model and also killed bacterial biofilms.
Targeted and Penetrative Delivery: The capsules can be delivered by a single, minimally invasive outpatient procedure.
Flexible and Versatile: The multi-layered capsule formulation allows for both immediate and sustained release of the drug precisely where it is required. The capsules can be adapted to deliver multiple other drug cargos to treat a variety of clinical indications.
Clinical Application
The principal area of clinical application on which the business is currently focused is targeted antibiotic delivery for recurrent and chronic urinary tract infections (UTI). UTI is one of the most common infectious diseases worldwide, affecting over 150 million patients per year globally and particularly the growing elderly population. The global market is estimated at US$10M per annum.
- UTI can be a particular problem in other vulnerable patient groups such as pregnant women, people with multiple sclerosis or spinal injury, in kidney transplant patients and in anyone requiring a urinary catheter
- UTI is one of the most common hospital-acquired infections, adding extra costs, complications and bed-days to patients suffering from other causes
- UTI is often recurring, causing much morbidity including kidney infection, sepsis and some mortality
- UTI is often difficult to diagnose and treat
- UTI can be caused by multiple species of bacteria, often in combination
- UTI bacteria can invade the bladder wall and persist in intracellular reservoirs or biofilms, which are resistant to antibiotics and the immune system, leading to recurrences or recalcitrance
AtoCap’s development of penetrating nano- or microcapsules may offer a solution to all of these problems – introduced directly into the bladder in a simple outpatient procedure. Our technology offers a unique way to repurpose already-approved therapeutics for a renewed utility.
The current approach to systemic antibiotic treatment is fraught with limitations:
- Bacterial intracellular invasion and possible biofilm formation can complicate treatment, as traditional antibiotics cannot access these microbes
- It is difficult to achieve high local doses in the bladder (many drugs are metabolised before this point) and without incurring systemic side effects
- Treatment may need to be prolonged, increasing the risk of antibiotic resistance, a global crisis which, if not addressed, is projected to cost USD100 trillion and 10 million people per year dying annually by 2050